- Home
- Electrochemical fuels
Electrochemical fuels
Carbon-based fuels make up the backbone of today's energy infrastructure and have unparalleled energy efficiency. If an efficient (photo-)electrochemical route to producing carbon-based fuels from CO2 can be developed, then a range of new primary energy options, including solar, wind, geothermal, hydro, and nuclear, will be capable of providing hydrocarbon fuels for our future energy infrastructure.
To enable the production of hydrocarbons in an electrochemical cell, the development of an efficient electrocatalyst to serve as the cathode is paramount. In the 1980s, copper was identified as a unique electrocatalyst material for its ability to produce hydrocarbons and alcohols with a faradaic selectivity in excess of 60%; however, the overpotential on copper electrodes is around 1 V. Since then, no other metal electrocatalysts have been found with selectivity as high as that of copper. The mechanism for how copper carries out this selective reduction, as well as the reason for the overpotential, have remained elusive.
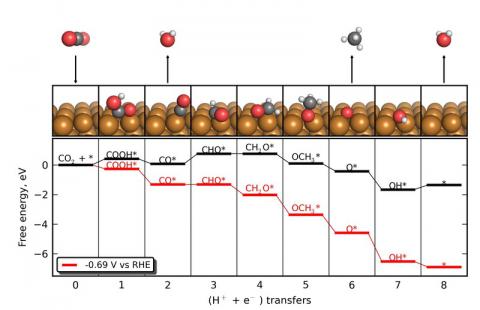
We are using density functional theory (DFT) to understand the reactivity and selectivity of the copper catalyst, and are using this knowledge to develop design principles that may enable more efficient electrocatalysts to be developed. The figure shows the elementary pathway that we have identified for this process, along with the corresponding free energetics of each elementary step as calculated with DFT. CO2 is first reduced by a proton-electron pair to form carboxy (COOH*), an adsorbate that bonds to the Cu electrocatalyst. (An asterisk, *, indicates a surface-adsorbed species.) The carboxy is then reduced to form CO*, liberating H2O in the process. In the potential-limiting step, CO* is protonated resulting in formyl (CHO*). This step is the most difficult from an energetic standpoint and determines the voltage requirements of the overall process. The formyl is further protonated to formaldehyde (CH2O*) and methoxy (CH3O*) before methane (CH4) is liberated, breaking the second C-O bond. This leaves O on the surface, which is cleared as water.
Using the findings of this proposed mechanism, we are studying the energetics of CO2 reduction on other metal electrocatalyst surfaces in order to understand trends in CO2 reduction from first principles. We are using the design principles that we are developing to search for candidate materials that can perform the electroreduction of CO2 with higher efficiency than Cu without sacrificing selectivity. As candidate materials are developed using computational tools, we will work with experimental collaborators to test these materials for their activity in CO2 reduction.
