It is notoriously difficult to electrochemically reduce N2 at ambient conditions. With such a process, one could use renewable electricity to make ammonia (NH3) for fertilizers efficiently at the point of use, or use the NH3 as a carbon-free hydrogen vector. From a thermodynamic perspective, electrochemical reduction is possible, but most attempts primarily produce hydrogen (H2) and very little NH3. We present a simple qualitative analysis to identify the origin of the problem. The analysis also points to strategies that may be employed to increase the NH3 selectivity substantially.
The competition between the hydrogen evolution (HER) and nitrogen reduction (NRR) is defined by the relative rate of the first step of each process. The first step of HER is the transfer of a proton to the surface (an electrochemical step), and the first step of NRR is the chemisorption of dissolved N2 (a chemical step). Because the NRR limiting potential on all known catalysts is quite negative, the first step of HER dominates, and the surface is H-covered. In this limit of low selectivity, the HER rate is first order in the concentration of proton-electron pairs, while the NRR rate is zeroth order.
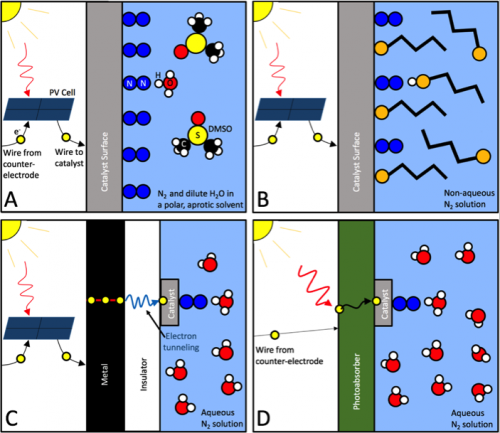
This model suggests that NRR selectivity can be enhanced by reducing the concentration of proton-electron pairs near the surface. We propose four possible strategies to accomplish this, outlined in Figure 1: (A) Limit the proton transfer rate by reducing the concentration of protons in the bulk solution. (B) Limit the proton transfer rate by increasing the barrier for proton transfer to the surface. (C) Limit the electron transfer rate by requiring electrons to tunnel through a thin insulator, taking care to prevent electron thermalization. (D) Limit the electron transfer rate by using photoabsorbers to supply a slow stream of electrons.