- Home
- Fuel cell catalysis
Fuel cell catalysis
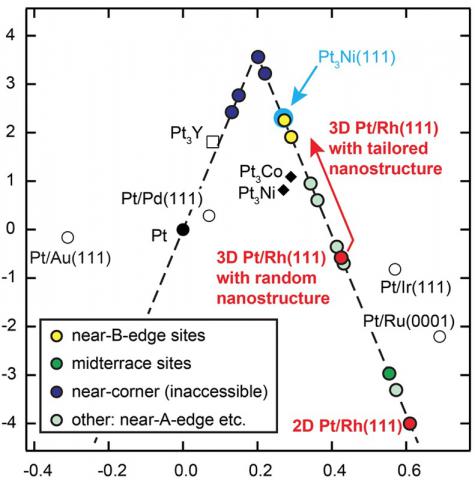
While the overall chemical reaction in a fuel cell is the same as in a combustion engine, its energy conversion is much more efficient since the reaction is separated in oxidation of the fuel at the anode, and the oxygen reduction reaction (ORR) at the cathode; the reaction free enthalpy is therefore directly converted into electricity. However, especially for the ORR an extremely careful design of catalyst materials is needed in order to meet two requirements: high catalytic activity to minimize costly use of its active component, Pt, and, at the same time, high stability to prevent catalyst degradation during long-term operation in a corrosive environment. At Suncat we attack these issues by investigating a range of different potential catalysts through combined theoretical and experimental screening.
