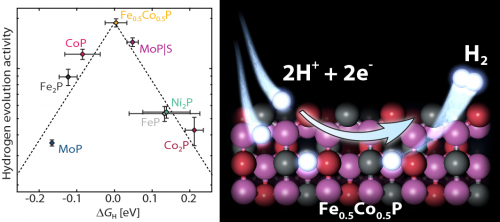
In order for hydrogen to be used as an energy carrier, it is necessary to have a sustainable way for producing hydrogen. The electrochemical hydrogen evolution reaction (HER) is one such route. This reaction is currently most effectively catalyzed by the Pt-group metals, which are expensive and scarce. Earth abundant alternatives that are comparable in catalytic activity are thus needed. Transition metal phosphides (TMPs) have emerged as highly active hydrogen evolution reaction (HER) catalysts. In this work, we synthesized different TMPs and correlated experimentally determined HER activities with the hydrogen adsorption free energies, ∆GH, calculated using density functional theory (DFT). We show that the TMPs follow a volcano relationship based on the hydrogen binding energies. Using our combined experimental-theoretical model, we predicted that the mixed metal TMP, Fe0.5Co0.5P, should have a near-optimal ∆GH. We synthesized and confirmed that indeed, Fe0.5Co0.5P exhibits the highest HER activity of the investigated TMPs. Our results show that it is possible to generate and verify a model of activity trends with predictive capabilities even for transition metal compounds with varied structures and surface terminations. The discovery of an optimized mixed metal TMP demonstrates how our model can be used for designing improved catalysts. Discrepancies in the volcano curve for TMPs and the volcano established for transition metals also point towards possible intrinsic differences between HER catalysis on pure metals and on the TMPs.
 Center for Interface Science and Catalysis
Center for Interface Science and Catalysis
A Partnership between SLAC & Stanford School of Engineering