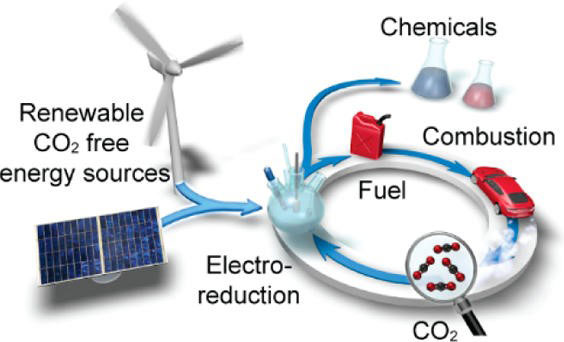
Electrochemical CO2 reduction into hydrocarbons and alcohols has the potential to enable a transition to a sustainable energy economy. Given that electrochemical processes operate under mild temperatures and pressures, electrochemical CO2 reduction is an ideal method for storing the energy from intermittent renewable sources. Currently, the major challenges for catalysis are activity, stability, and selectivity, and SUNCAT’s research program seeks to address all of these challenges. Theoretical and experimental efforts at SUNCAT work in synergy by utilizing a theory-experiment feedback loop to aid in both mechanistic understanding and materials discovery for electrochemical CO2 reduction (CO2R).
Our ongoing efforts include the mechanistic investigation of CO2 reduction on transition metals, descriptors for activity and selectivity for CO2 reduction over HER and for selectivity towards valuable C2 products. Our catalyst development efforts have focused on bimetallics, ionic compounds, single site catalysts. We use state-of-the-art theoretical methods in the determination of electrochemical pathways and the resultant kinetics, in order to determine descriptors of activity and selectivity. These descriptors are applied to computational screening of new materials for accelerated catalyst discovery. Experiments make use of latest methods in catalyst synthesis and electrochemical testing, and operando spectroscopic methods.