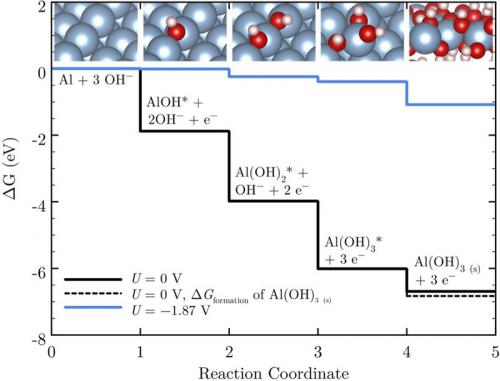
Free energy diagram for Al anodic dissolution at pH = 14.6 (equivalent to 4 M hydroxide electrolyte) on the stepped (211) surface. The black line shows dissolution at 0 V versus the standard hydrogen electrode (SHE), and the blue line shows dissolution at −1.87 V versus the SHE. The latter is the limiting potential for this facet and corresponds to the maximum open-circuit potential of this electrode in the current theoretical model. The dashed line provides the calculated formation energy of bulk Al(OH)3 as a reference.
There is much interest in developing alternative transportation energy sources due to the unfavorable climate change associated with the usage of fossil fuels. However, the practical specific energy (~1700 Wh/kg) of gasoline in an internal combustion engine is difficult to surpass with traditional energy storage methods. The Al-air battery is a favorable candidate, as it eliminates the need for cathode material storage and instead uptakes oxygen from the atmosphere, and thus possesses a promising maximum theoretical specific energy of 4140 Wh/kg. Moreover, the Al-air battery is projected to be less costly and safer than the Li-air battery due to its earth-abundance and compatibility with aqueous electrolytes.
Despite these favorable properties, the experimental open-circuit potential of the Al-air battery was reported to be only ~1.6 V. Researchers hypothesized that corrosion and surface film formation are the cause of this potential, and several groups reported an increased potential of ~1.8 V upon using corrosion-suppressing additives. In this study, we aimed to illuminate the fundamental limiting potential of the Al anode in order to decouple the possible effects of corrosion and film formation on the Al anode output potential. The anodic dissolution mechanism was examined by calculating the free energies of all reaction intermediates with density functional theory. The results revealed that only 77% of the total bulk thermodynamic energy contributed by the Al anode is available via the surface electrochemical pathway at open circuit, and this percentage drops to 63% upon drawing moderate current densities.
There are two contributors to this potential drop, as addressed in the following. (1) Asymmetry in multi-electron transfer reactions: in an ideal electrochemical reaction involving n-electrons, each electrochemical step should take up exactly 1/n of the total energy to ensure no energy is lost chemically at the limiting potential (the most negative potential at which no step along the reaction pathway is endergonic). However, at the limiting potential for all Al facets investigated in this study, the second and third electrochemical steps are exergonic and therefore a portion of the overall energy is lost chemically. (2) More importantly, structural differences between surface adsorbed intermediates and final bulk product: there is considerable chemical stabilization upon forming bulk Al(OH)3 from surface adsorbed Al(OH)3 units, resulting from the additional attractive interactions between neighboring Al atoms as well as between Al and OH (from a neighboring Al(OH)3 unit). This energy is also not available in the electrochemical pathway and corresponds to a loss of 14% to 29% of the total thermodynamic energy depending on the Al facet. The latter is a crucial point in general studies of battery electrochemistry since standard reduction tables are referenced from bulk thermodynamic formation energies, which may not always coincide with the energies of surface intermediates.