Adsorption models
An understanding of the breaking and making of chemical bonds at a solid surface is the starting point for any fundamental description of reactions at the solid-gas or solid-liquid interface. It is particularly important to understand which fundamental electronic structure properties of the surface determine its chemical reactivity.
The Newns-Anderson model and the socalled d-band model have proven particularly useful in the development of such an understanding. Adsorption on a transition metal surface occurs when an atom or a molecule (adsorbate) is bound in the vicinity of the surface. Depending on the strength of this bond a sharing of adsorbate and metal electrons will be involved and the energy levels of the adsorbate will be perturbed.
In the Newns-Anderson picture, the one-electron states on the adsorbate start to interact with all the valence states on the transition metal as it approaches the surface. These metal states form an almost continuous band of states and depending on the shape of these bands the interaction will result in a broadened single resonance if the metals states are broad and delocalized or split into bonding and anti-bonding resonances if the metal states are narrow and localized.
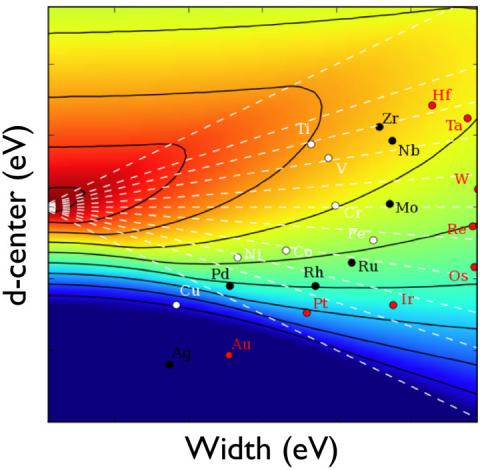
The latter gives rise to strong chemisorption. The figure shows an example of how the interaction energy between an adsorbate level and a semi-elliptic band behave as a function of the center and width of the semi-ellipse as calculated using the Newns-Anderson approach. A further simplification of this approach can be made. This is the so-called d-band model.
Since all the transition metals have a broad sp-band that is half-filled, then in accordance with the above this will lead to a broadened single resonance state. Assuming that this provides a constant contribution to the bond-strength and the problem is separable then one only has to worry about the interaction of this single resonance with the d-states of each metal. Since the filling of each metal is fixed then variations in the binding only depends on the position of the d-states relative to the Fermi-level if the d-states have a smooth behavior.
Since all the transition metals have a broad sp-band that is half-filled, then in accordance with the above this will lead to a broadened single resonance state. Assuming that this provides a constant contribution to the bond-strength and the problem is separable then one only has to worry about the interaction of this single resonance with the d-states of each metal. Since the filling of each metal is fixed then variations in the binding only depends on the position of the d-states relative to the Fermi-level if the d-states have a smooth behavior.
Recently, we have shown for a number of different systems that adsorbates with comparable electronic energy levels interact with the same surfaces in a similar way. The degeneracy of the energy levels now fully describes how different energies correlate and each line shift according to the surface structure (its reactivity). This means that we can determine the energetics of a reaction from much fewer calculations. Such models of reactivity trends that single out the important parameters describing the catalytic activity or selectivity are the essential prerequisites for the tailoring of surfaces with specified catalytic properties.
We utilize this type of fundamental electronic structure model to analyze and predict reactivity of metal alloys, ternary oxide surfaces, other compounds, and strained surfaces and nanoparticles.
