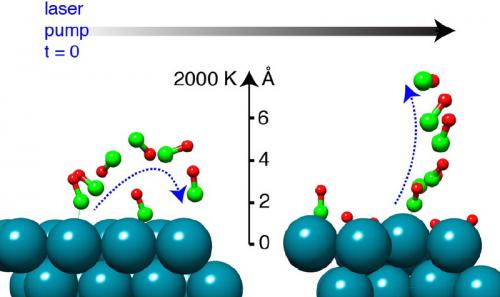
Rerouting bond-breaking pathways by coadsorbate interaction
Desorption of molecules from a surface into the gas phase is the most fundamental bond-breaking step in heterogeneous catalysis. In this process, the existence of a relatively short-lived and weakly-adsorbed precursor species has long been conjectured when interpreting measured kinetics. Recently, this surface species has been directly observed with a soft x-ray free-electron laser using ultrafast pump-probe techniques. The spectroscopic identification of the precursor state rationalizes many phenomena in gas-surface interactions and underpins our fundamental understanding of the kinetics of elementary surface reactions. In heterogeneous catalytic processes, many different species or promoters exist on the surface that can influence each other through adsorbate-adsorbate interactions. Effects of the coadsorbate interaction on desorption dynamics have, however, largely been unexplored and their role in the correlated chemical environment is presently still poorly understood.
In this study, we present experimental and theoretical evidence of a strong influence of coadsorbed oxygen atoms on the mechanistic aspects of CO desorption from Ru(0001). Using the ultrafast pump-probe technique based on x-ray absorption spectroscopy (XAS) using an x-ray free-electron laser together with a femtosecond optical laser pump and supported by density-functional theory (DFT) calculations, we show that CO desorption occurs via the direct pathway on oxygen-coadsorbed Ru(0001) rather than the precursor-mediated pathway found on bare Ru(0001). We found that the oxygen-induced reduction of the Pauli repulsion and increased electrostatic dative interaction between the CO 5σ and the positively charged Ru atom on 2O-CO/Ru(0001) drives the CO desorption via the direct desorption pathway instead of the precursor-mediated pathway. AIMD simulations further support the experimental observation and provide a microscopic view of surface bond-breaking processes. The fundamental insights gained here consolidate our understanding of surface chemical bonding and underline the importance of including coadsorbate interactions when unraveling dynamics of surface reactions.