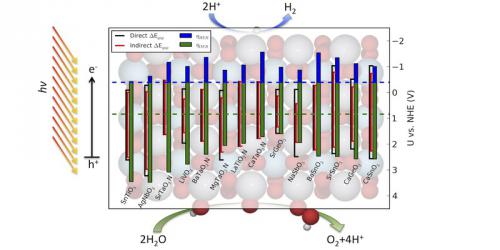
Theoretical overpotentials for the two reactions involved in H2O splitting. To catalyze water splitting with fast rates, a combination of the OER (green bar) and HER (blue bar) overpotentials should be contained within the black lines spanning the bandgap shown to the left of each overpotential bar.
Converting solar energy into chemical energy using photoelectrocatalytic water splitting is an attractive prospect for energy storage. However, suitable photoelectrocatalysts for performing this task have remained elusive to researchers for many years. In addition to being stable and cheap, water-splitting photoelectrocatalysts must have band gaps that allow them to absorb light in the solar spectrum readily and band edges that are properly positioned to catalyze the hydrogen evolution (HER) and oxygen evolution (OER) reactions. Furthermore, good photoelectrocatalysts should catalyze these reactions with low overpotentials in order to operate with high efficiencies.
In this work, we expand on a previous study that screened a dataset consisting of over 5000 perovskite oxides and oxynitrides in order to determine which of these materials had promising bandstructures for photoelectrocatalytic water splitting. Despite the large list of candidates, only 15 showed potential as photoelectrocatalysts. Taking these perovskites as a starting point, we calculated the theoretical overpotentials for each material, determining whether any of the materials also had the surface chemistry necessary to convert water into hydrogen and oxygen gas at high rates.
Unfortunately, our results suggest that none of these compounds should effectively serve as both photoabsorber and electrocatalyst. The theoretical overpotentials we determined were well outside of the energies provided to electron-hole pairs from absorbed photons, as shown in the above figure. With this insight, we advocate for a more modular approach to the design of new photoelectrocatalytic systems. Since single materials will likely not be capable of every requirement for photoelectrocatalytic systems, seeking out individual component materials and optimizing combinations of photoabsorbers and electrocatalysts is likely a much more effective strategy.