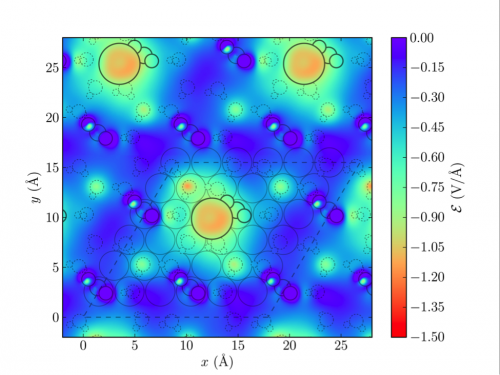
Electric field distribution taken at a z-slice near the center of *CO2 in a (6×6) unit cell with a K+ coverage of 1/36. One cell including metal atoms (parallelogram and solid circles in light gray) is outlined to illustrate the ion coverage. Solvent atoms are represented in dashed light gray circles. K+ (large circles) and *CO2 in its vicinity are outlined in black for emphasis.
Efficient electrochemical reduction of carbon dioxide (CO2) would simultaneously mitigate its effect on the climate, provide a sustainable, carbon-neutral means to store chemical energy, and produce feedstocks of carbon-containing molecules for the chemical industry. One of the simplest reduced products that can be made from CO2 is carbon monoxide (CO), which requires only two proton-electron transfers. Noble metals such as Ag and Au have been shown to be the best catalysts for this process. However, the process still requires a sizable overpotential and it has been hypothesized that the mechanism is limited by the endergonic reaction energy of the first intermediate, adsorbed CO2. Several experimental studies have also shown a pH effect, in which the overpotential for this reaction at pH 14 is lower than that at pH 7.
A mechanistic understanding of the CO2 reduction process is crucial to the design of more active catalysts. An ab initio investigation of the kinetics is very challenging due to the complexity of the electrochemical interface, where an accurate description of all components (ions, solvent, interfacial charge, and potential) is required. In this work, we use density functional theory (DFT) and an explicit model of the electrochemical interface to investigate the energetics of the CO2 to CO pathway on Ag(111). We show that, under realistic electrochemical conditions, the adsorbed CO2 intermediate is significantly stabilized by local electric fields exerted by partially solvated cations at the electrode | electrolyte interface. We develop a microkinetic model that incorporates electric field effects and electrochemical barriers determined through ab initio calculations, and show that this model accounts reasonably for the experimentally observed polarization curves.