Noble gas atoms are characterized by their inability to participate in chemical bonding. Still, dimers of these atoms exists. Already in 1930 Fritz London presented a theoretical explanation of the long-range attraction, now known as the London dispersion force. The attraction is caused by a mutual polarization of the electron densities of nonpolar interacting species. In the general case of neutral polar species, the dispersion force is part of the long-range intermolecular van der Waals (vdW) interaction.
In surface science and catalysis processes of physisorption, where only vdW interactions are responsible for the adsorption of molecular species on the catalyst, have been thoroughly investigated experimentally. In density functional theory (DFT), the most commonly used method in computational catalysis, the development of a satisfactory theoretical description of vdW interactions still poses a significant challenge. In a recent publication theorists from SUNCAT and the Danish Technical University presented the BEEF-vdW functional, designed to simultaneously describe long-range vdW interactions and short-range interactions related to chemical bonding. These capabilities enable modelling of an entire catalytic process involving physisorption steps as well as the transformation of chemical bonds assisted by the catalyst, while taking into account the vdW interactions in all stages of the process.
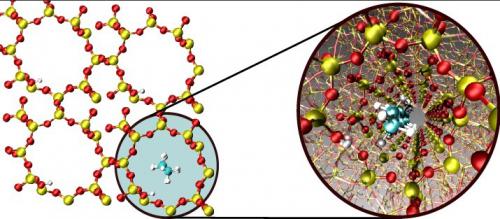
In zeolites vdW interactions can be even more pronounced than in conventional heterogeneous catalysts, since the molecules are exposed to a larger surface area inside a zeolite as compared to a two-dimensional metal surface. This molecular confinement is responsible for a catalytic activity of zeolites reminiscent of that of enzymes. Recently, researchers from SUNCAT and Haldor Topsøe showed that BEEF-vdW is capable of accurately describing such host-guest interactions in zeolites. The study focused on physisorption of linear alkanes inside the one-dimensional channels of zeolite H-ZSM-22. Most importantly for making predictions about trends, the slope of the linear relation between the physisorption enthalpy and the number of carbons atoms in the alkane is in excellent agreement with experiment. Furthermore, the experimentally measured enthalpies are reproduced within less than 10 kJ/mol, illustrating the high accuracy of the computational approach.
The excellent results have spurred additional efforts and modelling of guest-host interactions in more complex systems as well as catalytic reactions are currently conducted at SUNCAT.