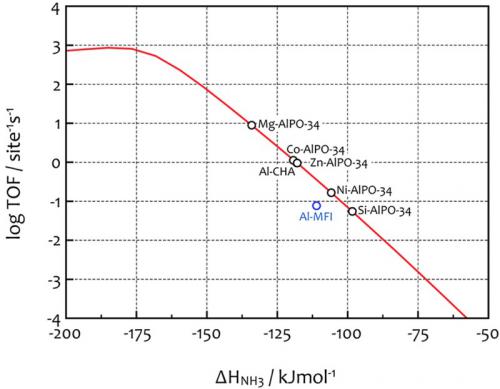
The relation between calculated ΔHads(NH3) and the simulated TOF for the methanol−propene reaction catalyzed by Me-CHA and Me-AlPO-34 materials. The calculated ΔHads(NH3) values of selected catalysts are highlighted.
Recent work has reported the discovery of metal surface catalysts by employing a descriptor-based approach, establishing a correlation between a few well-defined properties of a material and its catalytic activity. This theoretical work shows a similar approach in solid acid catalysis, focusing on the reaction between propene and methanol catalyzed by Brønsted acidic zeotype catalysts. Experimentally, the ammonia heat of adsorption is often used as a measure of the strength of acid sites. Using periodic DFT calculations, it is shown that this measure can be used to establish scaling relations for the energy of intermediates and transition states, effectively describing the reactivity of the acid site. This allows for the use of microkinetic modeling to predict a quantitative relation between the ammonia heat of adsorption and the rate of propene methylation from first principles. This study can be seen as the first step towards descriptor-based design of solid acid catalysts.