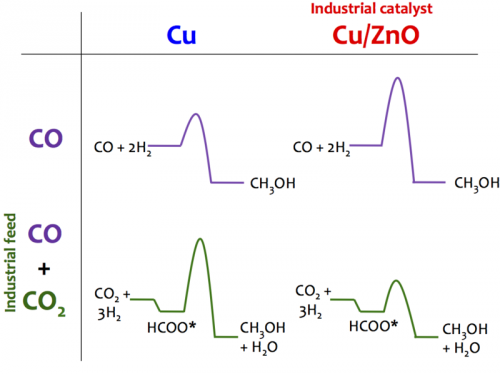
Revealing the mechanism: CO and CO2, on copper, can be hydrogenated to methanol, but not at the same time. The former reaction is poisoned by CO2 and requires a carbophilic Cu step, whereas the latter reaction requires a Zn promoter and proceeds as an oxophilic Cu/Zn step.
Methanol, an important chemical, fuel additive, and precursor for clean fuels, is produced by hydrogenation of carbon oxides over Cu-based catalysts. Despite the technological maturity of this process, the understanding of this apparently simple reaction is still incomplete with regard to the reaction mechanism.
Researchers at SUNCAT have integrated theoretical studies on different copper catalysts into a full micro-kinetic description of methanol synthesis. By teaming up with experimental researchers at the Fritz-Haber-Institute in Berlin they showed how the presence or absence of the Zn promoter dramatically changes not only the activity, but unexpectedly the reaction mechanism itself.
This Janus-faced character of Cu with two different sites for methanol synthesis, Zn-promoted and unpromoted, resolves the long-standing controversy regarding the Cu/Zn synergy and adds methanol synthesis to the few major industrial catalytic processes that are described on an atomic level.