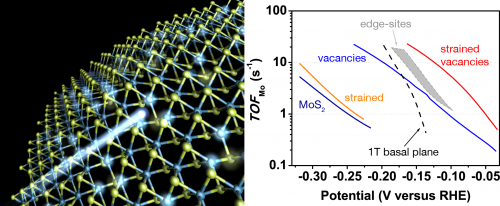
In order for hydrogen to become a viable energy carrier, the sustainable production of hydrogen is needed. One attractive possibility is to electrochemically generate hydrogen from water using energy directly from the sun or indirectly through wind power. To this end, an efficient catalyst for the hydrogen evolution reaction (HER) is needed. Interest in using molybdenum sulfide (MoS2) as an HER catalyst has skyrocketed in recent years due to its low-cost, good stability, and high activity. The minority edge-sites of two-dimensional layered MoS2 were first theoretically predicted1 and then experimentally proven2 to be the active sites for HER. Tremendous amounts of work have since gone into maximizing the density of edge-sites as well as optimizing their activity through nano-structuring techniques. However, few efforts have focused on activating the basal plane sites, which usually constitute the bulk of the MoS2 material. In our recent work accepted for publication in Nature Materials3, we combine theoretical predictions with experimental synthesis to show how new active sites for HER can be created directly in the basal plane through the formation of atomic sulfur vacancies. Elastic tensile strain and the concentration of vacancies are predicted to be effective tuning parameters for optimizing the HER activity. Continuous single layers of MoS2 were deposited on a substrate of nanocone arrays to generate strain, while argon plasma was subsequently used to generate vacancies. The optimized system demonstrated the highest intrinsic activity for HER amongst all MoS2-based catalysts synthesized to date. This new approach of activating the basal plane promises to make use of most of the bulk MoS2 material and provides a new route for creating highly active non-precious HER catalysts.
(1) Hinnemann, B.; Moses, P. G.; Bonde, J. L.; Jørgensen, K. P.; Nielsen, J. H.; Horch, S.; Chorkendorff, I.; Nørskov, J. K. Journal of the American Chemical Society. April 2005, pp 5308–5309.
(2) Jaramillo, T. F.; Jørgensen, K. P.; Bonde, J. L.; Nielsen, J. H.; Horch, S.; Chorkendorff, I. Science. July 6, 2007, pp 100–102.
(3) Li, H.; Tsai, C.; Koh, A. L.; Contryman, A. W.; Fragapane, A. H.; Zhao, J.; Han, H. S.; Manoharan, H. C.; Abild-Pedersen, F.; Nørskov, J. K.; Zheng, X. Nature Materials. 2015.