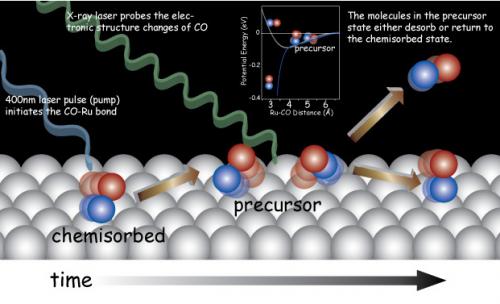
X-ray laser probes the electronic structure changes of CO molecules in desorption from Ru(0001) using X-ray emission spectroscopy and X-ray absorption spectroscopy. Upon exciting the substrate by using a femtosecond 400 nm laser pulse, molecules were pumped from the chemisorbed state to the precursor state. In the precursor state, the CO molecule is nearly free to rotate and to move parallel to the surface. The molecules in the precursor state either desorb or return to the chemisorbed state.
The adsorption and desorption of a molecule on a surface is one of the most fundamental chemical processes in interfacial chemistry. It has been proposed that adsorption and desorption proceed through a “precursor” state (1,2), in which a molecule is weakly bound to the surface. This is considered a transient state, in which the rotational and translational energy of the molecule is transferred from the surface before forming and to the surface after breaking a chemisorption bond. Though a large number of spectroscopic studies have been devoted especially to CO adsorption/desorption on metal surfaces, this “precursor” state has never before been directly observed.
The occupied and unoccupied valence electronic structure of adsorbed CO molecules on surfaces can be followed in an atom-specific way using soft X-ray emission spectroscopy and soft X-ray absorption spectroscopy, respectively (3). At SLAC, scientists have developed next-generation experimental setup at Stanford Synchrotron Radiation Laboratory (SSRL) to follow the electronic structure change in the bond breaking process of chemisorption bond. The chemisorption bond-breaking process involves both long range and short-range interactions between the molecule and the surface. The BEEF-vdW functional, which has been developed by SLAC scientists from SUNCAT and the Danish Technical University, simultaneously describes long-range van der Waals and short-range covalent bonding interactions in the frame work of density functional theory (4).
In this study, the short lived “precursor” state was probed by SLAC scientists, in collaboration with researchers from Stanford University; University of Hamburg, Center for Free-Electron Laser Science, Helmholtz-Zentrum Berlin for Materials and Energy, University of Potsdam and Fritz-Haber Institute of the Max Planck Society in Germany; Stockholm University in Sweden; and the Technical University of Denmark. They followed the electronic structure of CO molecules as their chemisorption state on Ru(0001) changed using ultra short x-ray pulses from the Linac Coherent Light Source (LCLS). They observed electronic structure changes that are consistent with a weakening of the CO interaction with the substrate,but without significant desorption. A large fraction of the molecules (30%) were trapped in a transient precursor state that preceded desorption. The team calculated the free energy of the molecules as a function of the desorption reaction coordinate using the BEEF-vdW density functional theory approach. Two distinct adsorption wells—chemisorbed and precursor state separated by an entropy barrier—explain the anomalously high prefactors often observed in desorption of molecules from metals.