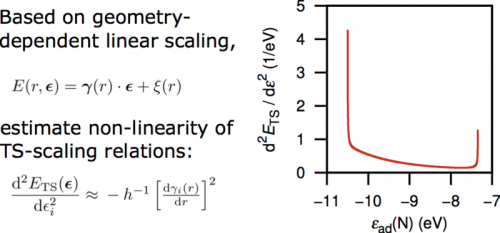
The dissociation of strong bonds in molecules shows large variations in the geometric structure of the transition state depending on the reactivity of the surface. It is therefore remarkable that the transition state energy can be accurately described through linear relations such as the Brønsted-Evans-Polanyi relations. Linear scaling relations for adsorbates with fixed structure can be understood in terms of bond order conservation but such arguments should not apply to transition states where the geometric structure varies. We have established a connection between linear adsorbate- and transition state scaling. The adsorbate scaling parameters vary smoothly through the transition state region from reactant to product, corroborating their interpretation as a measure of bond-order. This allows the prediction of transition state structure and energy based on the geometry-dependence of the scaling parameters, γ(r) and ξ(r). A predictive model has been derived that mainly reproduces classical linear transition state scaling. Analytically, we have found general constraints on the functional form of scaling relations, ETS (ε). To first order in the descriptor, ε, effects on the transition state energy resulting from change of the transition state geometry cancel. This can be interpreted as bond-order conservation where loss of intramolecular bonding is compensated by adsorbate-surface bonding. In general, the curvature of ETS(ε) is positive and is expected to be small in the intermediate ε-range that is neither very close to being completely up- or downhill. This region is where, according to the Sabatier principle, we expect the best catalysts. For N2 dissociation and related reactions we predict the highest deviation from linearity for very low activation barriers, which do generally not factor into the overall rate of catalysis. This justifies the use of linear scaling relations for transition state energies in the interpretation of the kinetics of chemical reactions.
 Center for Interface Science and Catalysis
Center for Interface Science and Catalysis
A Partnership between SLAC & Stanford School of Engineering