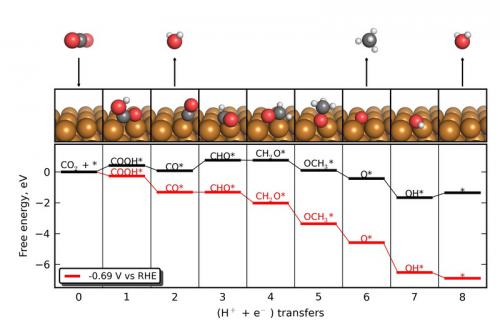
The reaction mechanism of the reduction of CO2 to CH4 on a Cu fcc(211) surface.
Chemical fuels, in particular hydrocarbons, have an unparalleled energy density and serve a key role in today's energy infrastructure as transportation fuels, heating fuels, and as chemical feedstocks. However,chemical fuels today are sourced only from fossil fuels and from biomass; while biomass provides a sustainable alternative to fossil fuels, its ultimate supply may be limited. If an efficient process could be developed that electrochemically transforms CO2 into hydrocarbon fuels, then any renewable or carbon-neutral energy source, including wind, solar, and nuclear, could be employed to make chemical fuels. We are employing electronic structure theory to understand and develop improved electrocatalysts for CO2 reduction. The figure shows the mechanism of the reduction of CO2 to methane on a copper surface, which is known to be a unique material in its ability to perform this reaction. In this figure, each frame represents the electrochemical addition of a proton/electron pair relative to the previous frame. The red curve shows the elementary free energy calculations at the calculated “onset” potential for the reaction. The conclusion is somewhat surprising: it is not the activation of CO2 that causes the process to be inefficient, but rather the subsequent reduction of adsorbed carbon monoxide (CO) that limits the potential requirement.
We have extended this analysis across a range of transition metals, and have found the elementary reduction of adsorbed CO to be stubbornly insensitive to catalyst material, partially explaining the dearth of electrode materials that have been found to be capable of reducing CO2 with a higher efficiency than copper. We are computationally screening hundreds of model alloy materials to attempt to find materials with these properties. If successful, this could lead to the development of catalysts that can achieve the production of hydrocarbons from CO2, potentially enabling future carbon-neutral fuels.