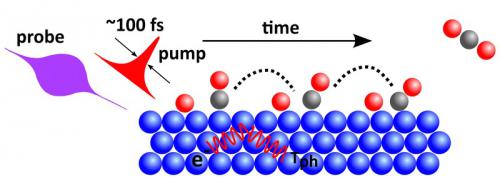
Schematics showing the pump-probe experiment inducing the formation of the O-CO surface bonds seen in the experimental spectra and reproduced in theory.
The transition state is the main component in the kinetic understanding of a chemical reaction. Molecules in the transition state have a very short lifetime and are therefore impossible to observe using standard characterization techniques. Ultrafast pump-probe techniques have, however, opened up opportunities for probing transition state structures by inducing a sufficient population of molecules in this region, which then subsequently can be detected on a very short timescale.
In this study it was demonstrated that ultrafast pump-probe x-ray spectroscopy based on the Linac Coherent Light Source (LCLS) x-ray free-electron laser can be used to probe the electronic structure of molecular species in the transition state region during CO oxidation on a Ru surface. An optical laser pump pulse to excite electrons in the substrate was used. In this process energy is immediately transferred to the adsorbate system, which leads to an increase in vibrational excitations of the system and population of the CO oxidation pathway on a picosecond timescale. To monitor the element specific time evolution of the empty valence electronic states O K-edge XAS was used during the process.
The results obtained, suggests an ultrafast energy transfer to the adsorbed O and CO from surface electrons excited by the laser pulse. Within the first picosecond new electronic states appeared, which clearly indicated the existence of new adsorbed species that did not resemble the separate systems in terms of CO molecules or O atoms adsorbed on the Ru surface. Through a comparison between experimental spectra and calculations these new resonances were identified as due to a shift in the positions of both CO and O but more importantly to the formation of a bond between CO and O that is substantially elongated in comparison to the CO2 final product. The potential energy surface obtained for the reaction identifies these species as OC-O fragments being in the near transition state region attempting to form CO2. A simple probability analysis based on the state distribution within the calculated potential energy surface provided an estimated ~10% population of species in the transition state region during the first few picosecond in good agreement with the experiments.